Contents
- Introduction
- Discovery/History
- Properties
- Uses


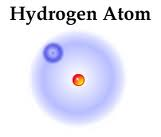


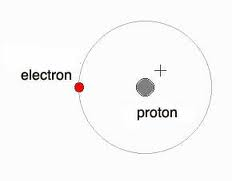
This is Hydrogen
Hydrogen is the most common element in the Universe. It makes up stars, molecular clouds, and many other compounds. Hydrogen has three natural isotopes: Protium (Normal Hydrgen), Deuterium (Heavy Hydrogen), and Tritium (which is very rare). Hydrogen is "lighter than air". Messing around with Hydrogen can be a lot of 25Kfun!
Hydrogen was first discovered by Henry Cavendish in 1776. He was a British scientist famous for his discovery of hydrogen.
Cavendish noticed that a gas was produced when zinc or iron was dropped into an acid. He called this gas "inflammable air". Which is what we now call hydrogen. Using his exacting experimental skills, Henry Cavendish was the first person to distinguish hydrogen from ordinary air. He also was the first to investigate its specific properties. Cavendish, like many before him, noticed that a gas was produced when zinc or iron was dropped into an acid. He called this gas "inflammable air" (known today as hydrogen). Using his exacting experimental skills, Cavendish was the first to distinguish this inflammable air from ordinary air and to investigate its specific properties. He presented a paper about his findings in 1766.
| Isotope | Atomic # | Atomic Mass | Melting Point | Boiling Point | Density |
|---|---|---|---|---|---|
| Hydrogen: | 1 | 1.008 | -259deg C | -253deg C | .09 |
| Deuterium, D2, "Heavy Hydrogen" | 1 | 2.0144 | -254.6deg C | 23.6K | -- |
| Tritium | 1 | 3.016 | -252.54deg C | 25K | -- |